

We Bring Elegance to Your Business
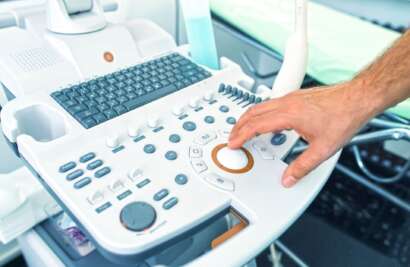
Maven was established in 2016 with a forward-thinking vision of being the ultimate service provider for medical device regulatory affairs, covering a wide range of global regulations. We aim to become a one-stop destination for all medical device manufacturers who place their trust in us to get things done. Our core service is technical documentation, which is complemented by other ancillary services we offer, allowing clients to avoid the need to engage multiple partners for different aspects related to product certification and registration.

We take care of your complex,
time-engaging regulatory tasks piled up in your schedule so that you can focus on vital business aspects
like Business expansion



We make your work so much easier
Let's Start Now
We are on a mission to redefine the consultation experience globally so let a team of 75 in-house consultants who have been exposed to industrial practices and trained on various regulatory requirements take care of your hurdles and make the experience effortlessly smooth.

Our Services Make Your Work Productive
We provide a sophisticated user experience and maintain close collaboration with our clients to deliver a tailored service.